The nitric acid method however produces toxic nitrogen dioxide. Upon the reaction with sulphuric acid it will change to a cyan blue.

How To Write The Net Ionic Equation For Cuo H2so4 Cuso4 H2o Youtube
Copper II oxide reacts with sulfuric acid to create water and copper II sulfate.

. Click to see full answer. However a reactive oxide layer is formed upon exposure to air. Hazards risks and precautions It is important in this practical activity to use.
What does magnesium oxide plus sulphuric acid make. The reaction between copper oxide and sulphuric acid. Copper oxide sulfuric acid copper sulfate water.
Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. CopperII oxide a black solid and colourless dilute sulfuric acid react to produce copperII sulfate giving a characteristic blue colour to the solution. We make copper sulfate from copper and sulfuric acid using two chemical methods and one electrochemical method.
Copper does not directly react with sulfuric acid so some form of oxidant is needed. CuO H2SO4 - CuSO4 H2O. Generally pure copper does not react with acetic acid.
Copper II oxide is a black solid which when reacted with sulphuric acid creates a cyan-blue coloured chemical called copper II sulfate. Updated 4 years ago. Students can then obtain blue copperII sulfate pentahydrate crystals.
The colour change you will see is black to blue as Copper oxide is usually found as a black powder. Copper oxide CuO and Sulphuric acid H2SO4 This answer does not mean that it is a safe thing to do. Here are the equations for those reactions.
What does lead and oxygen make. Warm acid will react faster than cold acid helping to make sure that all the acid reacts with the copper oxide. This layer interacts with non-oxidizing acids to give green copper II.
Copper sulphate is a form of salt that can be manufactured by the chemical reaction described in your question or it can be found in nature as a mineral in the form of chalcanthite bonattite or boothite. Using cupric oxide CuO copperII oxide a black powder itwill make hydrated copper sulfate CuSO45H2O -blue crystalsanything left. Black ink illustrator.
In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. What does sulfuric acid and copper oxide make. Copper oxide solid Sulphuric Acid aqueous- Copper Sulphate aqueous Water liquid In equation form.
Hydrogen peroxide and nitric acid are excellent oxidants and the first two methods demonstrate this. The second part of the name is sulfate so we need to use sulfuric acid. The reaction between copper oxide and sulphuric acid.
By reacting copperII oxide a black solid with colourless dilute sulfuric acid they produce copperII sulfate with a characteristic blue colour. Copper carbonate CuCO3 mixed with sulphuric acid H2SO4 produces copper sulphate CuSO4.
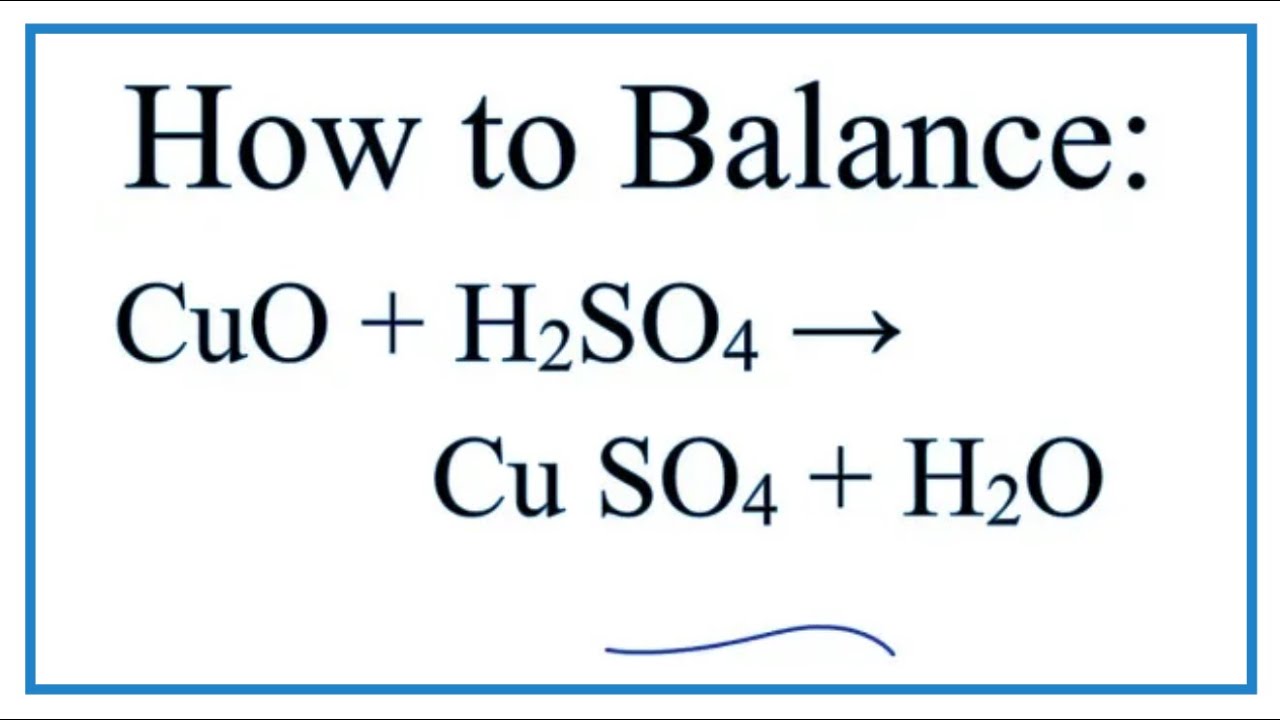
How To Balance Cuo H2so4 Cuso4 H2o Youtube
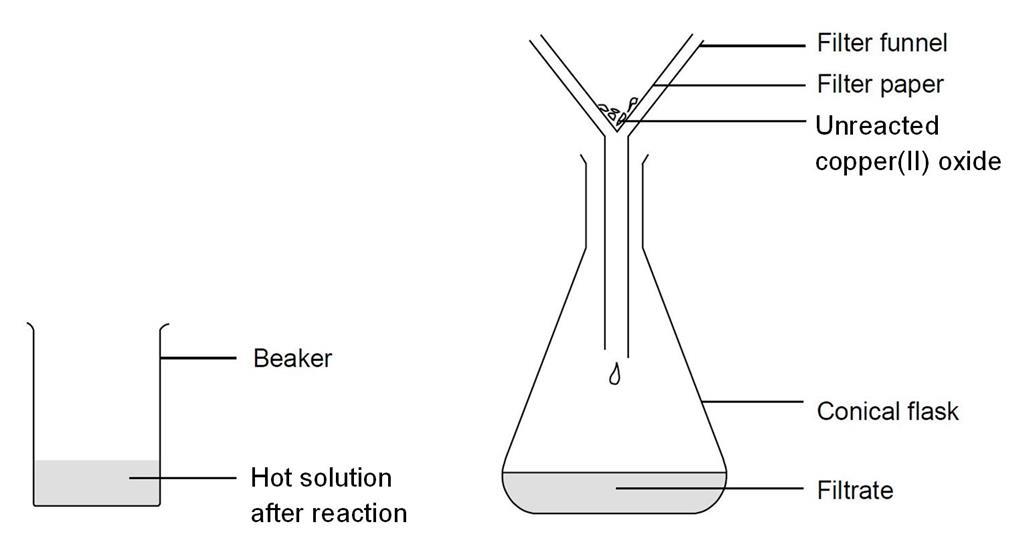
Reacting Copper Ii Oxide With Sulfuric Acid Experiment Rsc Education
0 Comments